Lamina Therapeutics pioneers a biomimetic approach to bone and cartilage regeneration in a single, synthetic polymer-based, entirely animal-free solution
Lamina.One Clinical Trial - CT2024-512977-28-01 - Study of the feasibility and safety of surgical implantation of an innovative therapy preparation combining a polymeric membrane impregnated with hydroxyapatite (the mineral salt of bone) with a hydrogel of autologous mesenchymal stem cells derived from bone marrow, for the treatment of an isolated osteocartilaginous lesion of the femoral knee - Monocentric Clinical trial Phase I starting March 2025 in Strasbourg, France for 6 patients
Our Technology
What we do ?
Lamina's Tech: A Game-Changer in Regenerative Medicine
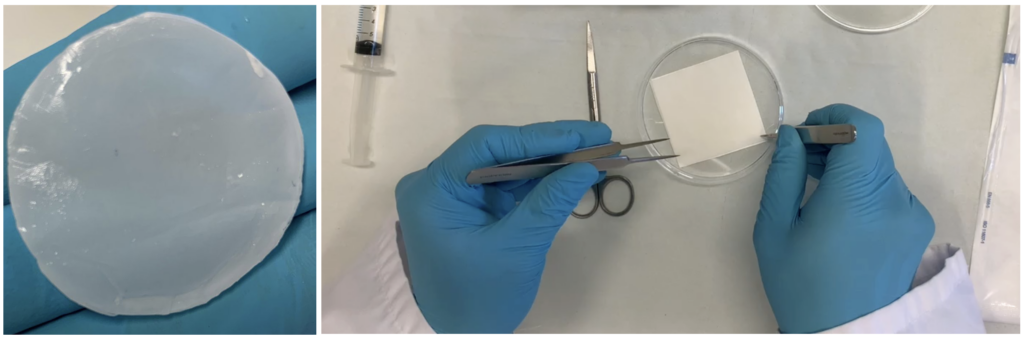
Lamina Therapeutics, a spin-off from INSERM (the French National Institute of Health and Medical Research) and the University of Strasbourg's Regenerative Nanomedicine unit, is revolutionizing bone and cartilage regeneration. Their flagship innovation, Lamina.One, is a fully synthetic, animal-free biomimetic solution that combines an implantable, biodegradable medical device with bone marrow-derived MSCs to promote tissue repair. The device stimulates bone regeneration, while the hydrogel-MSC mixture drives cartilage repair. Backed by strong preclinical results in mice, rats, and sheep models, as well as successful cadaveric feasibility studies, Lamina has secured EMA and ANSM authorization for a Phase I pilot clinical trial. This month, they are launching the trial in Strasbourg, marking a major milestone in their journey. Lamina Therapeutics is entering an exciting new phase, poised to transform regenerative medicine with cutting-edge, accessible solutions.
This pioneering approach heralds a paradigm shift in regenerative medicine, presenting a next-generation of implantable medical devices. Tailored to address orthopaedic, maxillofacial, and periodontal challenges, our innovative technique employs minimally invasive methodologies, promising transformative outcomes
Partnering
Lamina's Global Ecosystem Fuels Success

Technology
A Highly Innovative Breakthrough Living Medical Device Combining a Therapeutic Matrix and Autologous Stem Cells for Osteocartilaginous Regeneration in a Multinational Clinical Study Enhanced by AI-Driven Efficacy Imaging Analysis
The progressive breakdown of cartilage and subchondral bone remains a major challenge in orthopedic medicine, leading to chronic pain, reduced mobility, and a diminished quality of life for over 600 million patients around the world. Currently, market analysis reveals that no existing therapy provides a fully integrated regeneration of both cartilage and bone, a limitation that the Lamina.One® product overcomes through a novel breakthrough combined advanced therapeutic medicinal product (cATMP). Lamina.One® product is a highly innovative breakthrough approach in the field of osteoarthritis, addressing the urgent and unmet medical need for effective and long-lasting treatment solutions for knee osteochondral lesions. Lamina.One® product consists of a biodegradable polymeric membrane with hydroxyapatite for bone regeneration and an autologous mesenchymal stem cell hydrogel for cartilage repair. This first-in-the-world highly innovative breakthrough already authorized approach by EMA for a clinical trial is designed to facilitate osteoblast recruitment, support subchondral bone formation, and stabilize newly regenerated cartilage, ultimately restoring the whole structural integrity of the knee joint addressing a critical unmet medical need. With regulatory approval already secured from EMA and ANSM for a Phase I clinical trial in Strasbourg (France), we are now seeking funding to support a European Phase I/II clinical trial. Keywords: Highly innovative combined advanced therapy medicinal product (cATMP), implantable medical device, stem cells, knee osteoarthritis, dual regeneration of subchondral bone and cartilage, regenerative medicine, clinical trial
Partnering
Lamina Is Embedded In a Strong, Multinational Ecosystem To Accelerate The Company’s Success
LAMINA established strong collaborations with several industrial and academic partners. We are always interested in establishing new alliances and strategic partnerships to leverage our strengths in developing a next generation of regenerative products.
- Strasbourg University With as many as 6 Nobel laureates the university is ranked among the best in the League of European Research Universities - INSERM Inserm is the largest national public research institute in France focused on human health and medical research, ranked 2nd best research institution worldwide, according to the 2019 Scimago Institutions Ranking - Satt Conectus - NLC – The European Healthtech Venture Builder With experience of building and growing 44 ventures from the ground up, NLC has the expertise and network to support their ventures with each development step
News from Lamina
-

Creation of LAMINA THERAPEUTICS startup, fostered by SATT Conectus
https://www.conectus.fr/en/node/536
-

Big News from Spin-off Lamina Therapeutics and INSERM-University of Strasbourg UMR1260 Regenerative Nanomedicine !
We are thrilled to announce that Lamina Therapeutics has received approval from the European Medicines Agency (EMA) and the ANSM Agence nationale de sécurité du médicament et des produits de santé to initiate our first-in-human Phase I clinical trial in knee osteoarthritis! This groundbreaking trial, which will take place in Strasbourg, France, represents a world first: combining a therapeutic implantable medical device with autologous stem cells (combined ATMP) to treat advanced osteoarthritis by regenerating both subchondral bone and cartilage. Osteoarthritis remains a major challenge for millions of patients worldwide, and we are excited to take a leading role in pioneering this innovative solution to restore joint function and improve quality of life. We are deeply grateful to our exceptional team (Rana Smaida, Lemmens Stephan, Nadia Benkirane-Jessel...), collaborators, and regulatory partners who have worked tirelessly to make this possible. We’re looking forward to sharing updates as we begin this exciting journey towards transforming osteoarthritis treatment.
-

Lamina Therapeutics obtient l approbation de l EMA et de l ANSM
Nous sommes ravis de partager avec vous une bonne nouvelle ! Lamina Therapeutics a officiellement reçu l'approbation de l'Agence Européenne des Médicaments European Medicines Agency (EMA) et de l'ANSM Agence nationale de sécurité du médicament et des produits de santé pour lancer son premier essai clinique de phase I chez l'homme pour l'arthrose du genou, qui se déroulera à Strasbourg. Cet essai marque une première mondiale dans la combinaison d'un dispositif médical implantable thérapeutique combiné à des cellules souches autologues (médicament de thérapie innovante MTI combiné) pour traiter l'arthrose avancée en régénérant à la fois l'os sous-chondral et le cartilage. Cette approche pionnière offre un espoir à des millions de patients dans le monde qui souffrent de cette maladie. Cette belle avancée est le résultat du dévouement de notre équipe de recherche strasbourgeoise. Il s'agit d'une belle réussite pour Lamina Therapeutics fondée par Nadia Benkirane-Jessel, mais aussi pour le laboratoire INSERM UMR1260 Nanomédecine Régénérative. Cette belle collaboration entre les membres de Lamina Therapeutics plus particulièrement le CEO Lemmens Stephan, Project Manager Rana Smaida et les membres de l’UMR1260 est le secret de cette réussite. INSERM-University of Strasbourg UMR1260 Nanomédecine régénérative et Spin-Off Lamina Therapeutics
Address